Overview
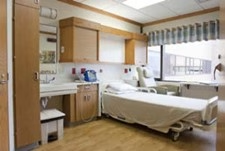 The Clinical Research Unit (CRU) is a dedicated research facility designed to provide medical scientists with the resources and conditions necessary to conduct clinical research in an effective and efficient manner. The CRU supports over 150 studies, including investigator-initiated and industry studies, representing all phases of clinical research, including Phase I (first in human) clinical trials. The CRU supports both outpatient and overnight stays for studies in adult populations ages 18 and over and is located on D6/6 in University Hospital in downtown Madison.
The Clinical Research Unit (CRU) is a dedicated research facility designed to provide medical scientists with the resources and conditions necessary to conduct clinical research in an effective and efficient manner. The CRU supports over 150 studies, including investigator-initiated and industry studies, representing all phases of clinical research, including Phase I (first in human) clinical trials. The CRU supports both outpatient and overnight stays for studies in adult populations ages 18 and over and is located on D6/6 in University Hospital in downtown Madison.
The CRU handles all phases of clinical trials and welcomes complex studies that may require Joint Commission certification and the ability to call an emergency code. If you plan to use the CRU, you should submit a free consult request at least one month before you submit your IRB application. Requests for feasibility reviews or Ancillary Service Report reviews will not be accepted until a CRU Consult has been submitted.
Scope of Services:
All RN staff in direct patient/participant care are BLS certified, chemotherapy certified, and trained in Human Subjects Protection and Good Clinical Practices.
- Research Subject Advocate (RSA)
- Evaluates all research study applications submitted to the CRU for safety.
- Works with principal investigators, study coordinators, and CRU nurses to ensure research is conducted in compliance with IRB and CRU approvals.
- Performs regular rounds with CRU nurse manager on study participants.
- Dietary Support:
- The CRU is supported by UW Health’s Nutrition services – meal trays are available to order for patient/participants required to fast prior to their visit (and visit includes additional study activities) and for longer visits that extend over a meal time.
- Limited Clinical Research Dietician services are available, such as to create study-specific menus for research protocols requiring certain meal specifications for study visits carried out on the CRU.
- CRU Sample Processing Lab (non-diagnostic):
- Located on the unit and supported by CRU staff.
- Secured via badge access only, limited to CRU staff.
- Able to support simple processing of research samples.
- Includes room temp and refrigerated centrifuges, biosafety cabinet, a lab grade refrigerator, -20- and -80-degrees Celsius freezers on Primex temperature monitoring system with alarm for temporary sample storage.
- Note: CRU does not ship samples.
- CRU Space:
- UW Health Clinical Trials Tour – CRU
- 15 private rooms with a bed and private bathroom that can be flexed to support outpatient or overnight stays:
- Two are ADA accessible
- Two are designed to support negative airflow isolation
- Four are hardwired for bedside cardiac monitoring
- One private outpatient/clinic type room and a four-bay outpatient treatment area.
- All rooms have oxygen and suction capabilities and can support patient/participants on telemetry (i.e., cardiac monitoring).
- The CRU is in the footprint of University Hospital (UH) and is considered clinical space:
- UH provides additional oversight and support and as such, the CRU is required to follow all applicable hospital and regulatory policies (e.g., The Joint Commission [TJC] and Center for Medicare & Medicaid Services [CMS]).
- There is a crash cart stored on the unit and the CRU is supported by UW Health’s Emergency Response Teams (e.g., Code Blue, Rapid Response, and Medical Response).
- All patients/participants seen on the CRU are required to have a Medical Record Number (MRN). If you will be enrolling participants who are not patients of UW Health, you will need to request an MRN be created for them. Their medical record will include their CRU appointment, provider orders, and visit documentation such as nursing and physician notes, procedure notes, medication documentation, vital signs, lab results (if resulted by UW Health labs), etc. Please refer to the Documentation of Clinical Research Related Care resource for additional details.
Publications arising from any research project receiving support from the UW ICTR, regardless of funding source, must acknowledge support by stating:
“The project described was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR002373. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.”
PLEASE NOTE: ICTR support includes not only direct fiscal support such as funding for pilots, but also ICTR resources like scientific editing, use of the Clinical Research Unit, CRU consults, ICTR Scientific Review Committee review, and consultations in biostatistics.

